Ion exchange membranes as electrolyte for high performance Li-ion batteries
Abstract
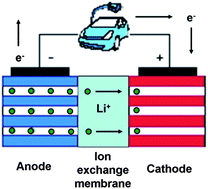
High ionic conductivity exceeding 10−3 S cm−1 at room temperature is achieved with lithiated perfluorinated sulfonic acid (PFSA-Li) ion exchange membranes by swelling in nonaqueous organic

 Please wait while we load your content...
Please wait while we load your content...