Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction†
Abstract
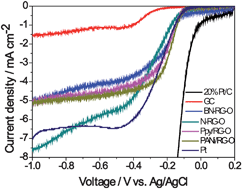
We present two different ways to fabricate nitrogen-doped graphene (N-graphene) and demonstrate its use as a metal-free catalyst to study the catalytic active center for the oxygen reduction reaction (ORR). N-graphene was produced by annealing of graphene oxide (G-O) under ammonia or by annealing of a N-containing polymer/reduced graphene oxide (RG-O) composite (polyaniline/RG-O or polypyrrole/RG-O). The effects of the N precursors and annealing temperature on the performance of the catalyst were investigated. The bonding state of the N atom was found to have a significant effect on the selectivity and catalytic activity for ORR. Annealing of G-O with ammonia preferentially formed graphitic N and pyridinic N centers, while annealing of polyaniline/RG-O and polypyrrole/RG-O tended to generate pyridinic and pyrrolic N moieties, respectively. Most importantly, the electrocatalytic activity of the catalyst was found to be dependent on the graphitic N content which determined the limiting current density, while the pyridinic N content improved the onset potential for ORR. However, the total N content in the graphene-based non-precious metal catalyst does not play an important role in the ORR process.

 Please wait while we load your content...
Please wait while we load your content...