Lithium metal fluorosulfate polymorphs as positive electrodes for Li-ion batteries: synthetic strategies and effect of cation ordering†
Abstract
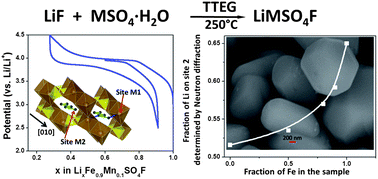
Transition-metal fluorosulfates are currently being extensively explored for their use as cathodes in Li-ion batteries. Several new polymorphs of LiMSO4F (M = Fe, Mn, Zn) crystallizing in the tavorite, triplite and sillimanite structures have captured much recent interest, but synthetic access is limited and the underlying phase stability and ion transport in these materials are poorly understood. Here we report that solvothermal routes to LiMSO4F (M = Fe, Mn, Zn) offer significant advantage over both exotic ionothermal methods and solid state synthesis by enabling greater control of the chemistry. We show new limits for the onset of triplite

 Please wait while we load your content...
Please wait while we load your content...