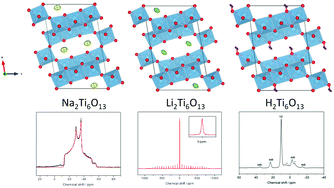
Li2Ti6O13 and H2Ti6O13 were easily synthesized from Na2Ti6O13 by successive Na+–Li+–H+ ion exchange. The crystal structures of Na2Ti6O13, Li2Ti6O13 and H2Ti6O13 were investigated using neutron powder diffraction. Monovalent A+ cations (Na, Li and H) have been located using difference Fourier analysis. Although monoclinic lattice parameters (space group C2/m) of the three titanates remain almost unchanged with retention of the basic [Ti6O132−] network, monovalent Na, Li and H cations occupy different sites in the tunnel space. By comparing the structural details concerning the A+ oxygen coordination, i.e. NaO8 square prismatic coordination, LiO4 square planar coordination and covalently bond H atoms, with results from 23Na, 7Li and 1H NMR spectroscopy we were able to obtain a more detailed insight into the respective local distortions and anharmonic motions. We were able to show that the site that the A+ cation occupies in the hexatitanate channel structure strongly influences the lithium insertion properties of these compounds and therefore their usefulness as electrode materials for energy storage.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...