Ruthenium cryptates with an unusual selectivity for nitrate†
Abstract
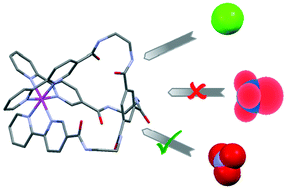
The synthesis of two new tripodal complexes [Ru(L3)](PF6)2 and [Ru(L4)](PF6)2, encapsulating a ruthenium(II) cation, has been successfully achieved and the products fully characterized, including by X-ray structural determination. The smaller cavity, built around a tris(2-aminoethyl)amido scaffold demonstrated only moderate and predictable interactions with a range of anions and no significant spectroscopic change with nitrate, chloride and bromide, although dihydrogen phosphate did result in an almost stoichiometric precipitation. The expansion of the cavity to include the more rigid 1,3,5-benzenetricarbonylamide group creates a larger cavity, which shows a decrease in the emission on the introduction of chloride, bromide, hydrogen sulfate and nitrate salts, with the 1H NMR titrations giving a surprisingly high binding affinity for nitrate over the smaller and simpler halides.

 Please wait while we load your content...
Please wait while we load your content...