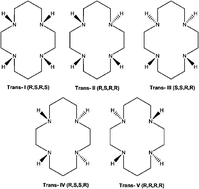
Ruthenium complexes including nitrosyl or nitrite complexes are particularly interesting because they can not only scavenge but also release nitric oxide in a controlled manner, regulating the NO-level in vivo. The judicious choice of ligands attached to the [RuNO] core has been shown to be a suitable strategy to modulate NO reactivity in these complexes. In order to understand the influence of different equatorial ligands on the electronic structure of the Ru–NO chemical bonding, and thus on the reactivity of the coordinated NO, we propose an investigation of the nature of the Ru–NO chemical bond by means of energy decomposition analysis (EDA), considering tetraamine and tetraazamacrocycles as equatorial ligands, prior to and after the reduction of the {RuNO}6 moiety by one electron. This investigation provides a deep insight into the Ru–NO bonding situation, which is fundamental in designing new ruthenium nitrosyl complexes with potential biological applications.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...