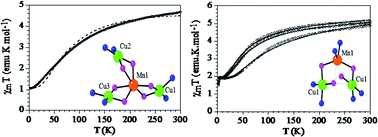
A tetra-nuclear, star-shaped hetero-metallic copper(II)–manganese(II) complex, [{CuL(H2O)}2(CuL)Mn](ClO4)2 (1) has been synthesized by reacting the “complex as ligand” [CuL] with Mn(ClO4)2 where H2L is the tetradentate di-Schiff base derived from 1,3-propanediamine and 2-hydroxyacetophenone. Upon treatment with the polyatomic anions azide, cyanate, or thiocyanate in methanol medium, complex 1 transforms into the corresponding trinuclear species [(CuL)2Mn(N3)2] (2), [(CuL)2Mn(NCO)2] (3) and [(CuL)2Mn(NCS)2] (4). All four complexes have been structurally and magnetically characterized. In complex 1 the central Mn(II) ion is encapsulated by three terminal [CuL] units through the formation of double phenoxido bridges between Mn(II) and each Cu(II). In complexes 2–4 one of the CuL units is replaced by a couple of terminal azide, N-bonded cyanate or N-bonded thiocyanate ions respectively and the central Mn(II) ion is connected to two terminal Cu(II) ions through a double asymmetric phenoxido bridge. Variable temperature magnetic susceptibility measurements show the presence of moderate ferrimagnetic exchange interactions in all the cases mediated through the double phenoxido bridges with J values (H = −JSiSi + 1) of −41.2, −39.8 and −12.6 cm−1 (or −40.5 and −12.7 cm−1 if we use a model with two different exchange coupling constants) for the tetranuclear MnCu3 cluster in compound 1 and −20.0, −17.3 and −32.5 cm−1 for the symmetric trinuclear MnCu2 compounds 2–4. These ferrimagnetic interactions lead to spin ground states of 1 (5/2 − 3*1/2) for compound 1 and 3/2 (5/2 − 2*1/2) for compounds 2–4.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...