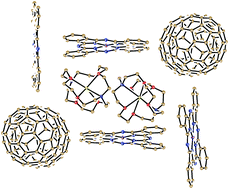
Ionic compounds containing the anion of iron phthalocyanine (FeIPc)−, {(FeIPc)−}·(cryptand[2,2,2]·[Na+])·(C6H4Cl2) (1), and the fullerene C70− and FeIPc− anions, (C70−)2·{(FeIPc)−}6·(cryptand[2,2,2]·[Na+])8·(C6H4Cl2)8.65·(C6H14)0.35 (2), were obtained as single crystals. Their crystal structures and optical and magnetic properties were analyzed. Spectra of 1 and 2 manifest intense bands in visible-NIR range at 820, 690, 555, 528, 449, 400 and 316 nm attributed to (FeIPc)−. The four-coordinate (FeIPc)− anions show an EPR signal with perpendicular and parallel components at g⊥ = 2.487 and g‖ = 2.353 in 1 and g⊥ = 2.328 and g‖ = 2.230 in 2. According to magnetic measurements the (FeIPc)− anions have low spin (S = 1/2) state indicating d7 configuration for the FeI atoms with the odd electron on the dz2 orbital. Fullerene anions form singly bonded (C70−)2 dimers in 2, the shape of which efficiently fitted with the large planar phthalocyanine anions to be surrounded completely by eight (FeIPc)− anions. The (C70−)2 dimers are diamagnetic and EPR silent up to 350 K. Both complexes contain channels formed by four (FeIPc)− planes in 1 or four (FeIPc)− planes and two (C70−)2 dimers in 2. The channels are occupied by double chains of alternating cryptand[2,2,2]·[Na+] cations and solvent C6H4Cl2 molecules.

 Please wait while we load your content...
Please wait while we load your content...