DFT study on the mechanism of water-assisted dihydrogen elimination in group 6 octahedral metal hydride complexes†
Abstract
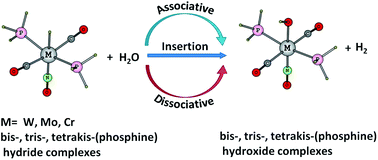
The reaction of water with octahedral bis-, tris- and tetrakis-(phosphine)tungsten, (phosphine)molybdenum and (phosphine)chromium complexes has been studied using B3LYP/def2-TZVPP level of DFT to elucidate dissociative, associative and hydride migratory insertion mechanisms for hydrogen elimination. In the dissociative mechanism, phosphine dissociation requires 19.3–28.5 kcal mol−1 of energy. The phosphine–water ligand exchange is endergonic due to poor coordination ability of water to group 6 metals (binding energy 8.8–15.5 kcal mol−1). The ligand exchange leads to intermolecular M–H⋯H2O dihydrogen interaction and facilitates dihydrogen elimination (Gact = 6.8–15.5 kcal mol−1). In the associative mechanism, a water molecule in the first solvation shell interacts with the M–H bond through a dihydrogen bond (interaction energy 2.7–4.0 kcal mol−1) and leads to the elimination of H2 by forming a hydroxide complex. Compared to the dissociative mechanism, Gact of associative mechanisms are ∼22 kcal mol−1 higher. In the hydride migratory insertion mechanism, the hydride ligand shifts to the CO ligand (Gact = 25.4–30.4 kcal mol−1) to afford a formyl complex and subsequently the H–H bond coupling occurs between formyl and water ligand (Gact = 2.8–4.4 kcal mol−1). In many cases, the migratory insertion mechanism can simultaneously operate with the dissociative mechanism as a minor pathway, whereas owing to high Gact value, the associative mechanism can be described as inactive in the reaction. The general argument that dihydrogen elimination is preceded by the formation of a dihydrogen intermediate is not applicable for the systems studied herein as the most favoured dissociative mechanism does not pass through such an intermediate. On the other hand, irrespective of the mechanisms, dihydrogen elimination invariably occurs with the formation of a dihydrogen bonded transition state. Our results also suggest that group 6 octahedral metal hydride complexes are attractive targets for the design of water splitting reactions.

 Please wait while we load your content...
Please wait while we load your content...