Organic–inorganic hybrid materials based on iron(iii)-polyoxotungstates and 1-butyl-3-methylimidazolium cations†
Abstract
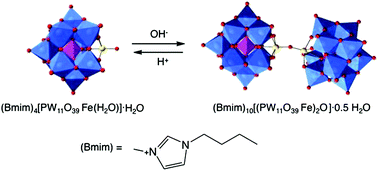
The iron(III) μ-oxo bridged dimeric polyoxometalate [(PW11O39Fe)2O]10− was isolated by reacting the transition metal monosubstituted Keggin anion [PW11O39Fe(H2O)]4− and the ionic liquid 1-butyl-3-methylimidazolium bromide, (Bmim)Br, at pH 5.5. The crystal structure of (Bmim)10[(PW11O39Fe)2O]·0.5H2O (1) (monoclinic, space group P21/n, Z = 4) was determined by single crystal X-ray diffraction. By changing the reaction conditions, (Bmim)4[PW11O39Fe(H2O)]·H2O (2) was obtained, whilst the reaction between the Bmim+ cation and the heteropolyanion [SiW11O39Fe(H2O]5−, in the pH conditions used for 1, afforded (Bmim)5[SiW11O39Fe(H2O)]·4H2O (3). The compounds were characterized by spectroscopic techniques, thermal analysis, cyclic voltammetry, magnetic measurements and mass spectrometry. This study contributes to the understanding of iron μ-oxo dimer formation in polyoxometalate chemistry and calls attention to the influence of the counter-cations on the stability and formation of compound 1. The combination of the cationic part of ionic liquids and iron-substituted polyoxotungstates is predicted to lead to new materials with interest to catalysis, electrocatalysis and ionic liquid based nanocomposites.

 Please wait while we load your content...
Please wait while we load your content...