Functions of MgH2 in hydrogen storage reactions of the 6LiBH4–CaH2 reactive hydride composite†
Abstract
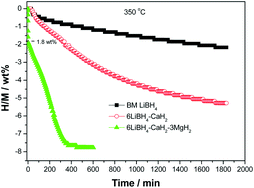
A significant improvement of hydrogen storage properties was achieved by introducing MgH2 into the 6LiBH4–CaH2 system. It was found that ∼8.0 wt% of hydrogen could be reversibly stored in a 6LiBH4–CaH2–3MgH2 composite below 400 °C and 100 bar of hydrogen pressure with a stepwise reaction, which is superior to the pristine 6LiBH4–CaH2 and LiBH4 samples. Upon dehydriding, MgH2 first decomposed to convert to Mg and liberate hydrogen with an on-set temperature of ∼290 °C. Subsequently, LiBH4 reacted with CaH2 to form CaB6 and LiH in addition to further hydrogen release. Hydrogen desorption from the 6LiBH4–CaH2–3MgH2 composite finished at ∼430 °C in non-isothermal model, a 160 °C

 Please wait while we load your content...
Please wait while we load your content...