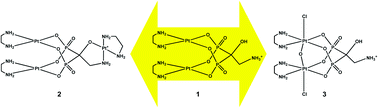
Geminal bisphosphonates (BPs), used in the clinic for the treatment of hypercalcaemia and skeletal metastases, have been also exploited for promoting the specific accumulation of platinum antitumor drugs in bone tissue. In this work, the platinum dinuclear complex [{Pt(en)}2(μ-AHBP-H2)]+ (1) (the carbon atom bridging the two phosphorous atoms carrying a 2-ammonioethyl and a hydroxyl group, AHBP-H2) has been used as scaffold for the synthesis of a Pt(II) trinuclear complex, [{Pt(en)}3(μ-AHBP)]+ (2), and a Pt(IV) adamantane-shaped dinuclear complex featuring an oxo-bridge, [{PtIV(en)Cl}2(μ-O)(μ-AHBP-H2)]+ (3) (X-ray structure). Compound 2 undergoes a reversible, pH dependent, rearrangement with a neat switch point around pH = 5.4. Compound 3 undergoes a one-step electrochemical reduction at Epc = −0.84 V affording compound 1. Such a potential is far lower than that of glutathione (−0.24 V), nevertheless compound 3 can undergo chemical reduction to 1 by GSH, most probably through a different (inner-sphere) mechanism. In vitro cytotoxicity of the new compounds, tested against murine glioma (C6) and human cervix (HeLa) and hepatoma (HepG2) cell lines, has shown that, while the PtIV dimer 3 is inactive up to a concentration of 50 μM, the two PtII polynuclear compounds 1 and 2 have a cytotoxicity comparable to that of cisplatin with the trinuclear complex 2 generally more active than the dinuclear complex 1.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...