A ferrocenyl-guanidine derivative as a highly selective electrochemical and colorimetric chemosensor molecule for acetate anions†
Abstract
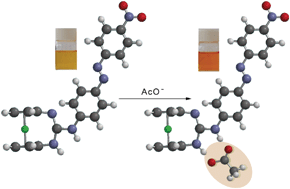
A highly preorganized chemosensor molecule 1 based on a ferrocenyl-guanidine decorated with a chromogenic aryl azo moiety recognizes the acetate anion in acetonitrile solution. At first, receptor 1 underwent two-step oxidation events. Initially, oxidation of 1 occurs at the Fe(II) centre (Ep = 440 mV) to form a ferrocenium species, followed by fast electron transfer from the guanidine moiety of the receptor to the Fe(III) centre with concomitant generation of an Fe(II) species with a radical cation centred at the nitrogen atom. In the second step, the radical cation species formed should undergo electrochemical oxidation at higher potential (Ep = 830 mV). This assumption is supported by spectroelectrochemical studies. A remarkable cathodic shift (182 mV) of the ferrocene/ferrocenium oxidation peak (Ep = 440 mV) and a progressive red-shift (Δλ = 30 nm) of the low energy band are observed in its absorption spectrum upon complexation of receptor 1 with the acetate anion. This change in the absorption spectrum is accompanied by a colour change from yellow to orange, which can be used for the “naked-eye” detection of this anion. Its monoprotonated form is able to selectively sense the less basic Cl−, Br−, NO3−, and HSO4− anions: the oxidation redox peak at Ep = 865 mV is cathodically shifted (107–182 mV).

 Please wait while we load your content...
Please wait while we load your content...