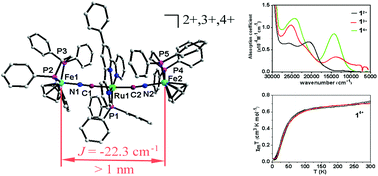
Treatment of trans-(Ph-tpy)Ru(PPh3)(CN)2 (Ph-tpy = 4′-phenyl-2,2′:6′,2′′-terpyridine, PPh3 = triphenylphosphine) with 2 equiv of Cp(dppe)Fe(NCCH3)Br (dppe = bis(diphenylphosphino)ethane) in the presence of NH4PF6 produced a trinuclear cyanide-bridged complex, trans-[Cp(dppe)Fe(CN)(Ph-tpy)Ru(PPh3)(CN)Fe(dppe)Cp][PF6]2 (1[PF6]2). Its one-electron oxidation product (1[PF6]3) and two-electron-oxidation product (1[PF6]4) were obtained by oxidation with (Cp)2FePF6 and AgPF6, respectively. Firstly, the crystal structures of the cyanide-bridged complexes with three stable states were fully characterized. The reversible electrochemistry measurement of 12+2+ shows the presence of a long range intervalence interaction between the external iron centres. Both 13+3+ and 14+4+ were considered to be Class II mixed valence complexes according to the classification of Robin and Day. Magnetic analysis indicated the presence of a moderately strong antiferromagnetic coupling between the two remote Fe(III) ions across the Fe–NC–Ru–CN–Fe array in 14+4+. This proves that the Ru(II)-dicyano complex is a bridging ligand that can transmit electro- and magneto-communication.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...