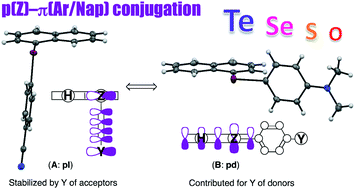
Magnitudes of the p(Z)–π(Ar/Nap) conjugation were evaluated for 1-(arylchalcogena)naphthalenes (1-(ArZ)Nap, 1-(p-YC6H4Z)C10H7; 1 (Z = Te), 2 (Se), 3 (S) and 4 (O)). Structures of 1 were determined by X-ray analysis for Y = NMe2 (b), OMe (c) and CN (i). For 1b and 1c that have electron donating Y, the Z–CAr bond is located on the naphthyl plane with Z–CNap being perpendicular to the aryl plane, which we define as (B: pd). On the other hand, the structure of 1i with electron donating Y is (A: pl), of which Z–CAr is placed almost perpendicular to the naphthyl plane with Z–CNap being located on the aryl plane. Each structure of 1a (Y = H), 1b, 1c, 1d (Me), 1e (F), 1f (Cl), 1g (Br), 1h (COOEt), 1i and 1j (NO2) was determined by NMR in chloroform-d. Structures of 1 in the solutions are (B: pd) for b, c and e that have electron donating Y, (A: pl) for f–j with electron accepting Y, and in equilibrium between (B: pd) and (A: pl) for a and d of which Y are rather neutral. The results for 2–4 are very similar to those of 1 in solutions. Quantum chemical calculations were performed on 1–4 with Y of a, b′ (NH2), d, f and j. Magnitudes of the p(Z)–π(Ar/Nap) conjugation were well-evaluated by NBO (natural bond orbital) analysis. The values were 12.6 and 13.0 kcal mol−1 for the typical forms of (A: pl) and (B: pd) of 1a, respectively, resulting in a much smaller energy difference between the two (0.4 kcal mol−1), which should correspond to the observed result. It is well-demonstrated that the p(Te)–π(Ar/Nap) conjugation operates effectively in 1, although the magnitudes increase in the order of Z = Te < Se < S < O. Thermal effect of the Gibbs free energies is shown to play an important role in the energy profiles of 1a–4a.

 Please wait while we load your content...
Please wait while we load your content...