The prevalence of isocloso deltahedra in low-energy hypoelectronic metalladicarbaboranes with a single metal vertex: manganese and rhenium derivatives†
Abstract
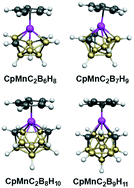
Theoretical studies on the hypoelectronic metalladicarbaboranes CpMC2Bn−3Hn−1 (M = Mn, Re; n = 9, 10, 11) having 2n skeletal electrons indicate that true isocloso MC2Bn−3 deltahedra are highly energetically favored in which the metal atom occupies the single degree 6 vertex. This contrasts with the previously studied isoelectronic diferradicarbaboranes Cp2Fe2C2Bn−3Hn−1 for which the isocloso structure is clearly favored only for the 10-vertex system. For the 12-vertex hypoelectronic manganadicarbaborane CpMnC2B9H11 with 2n (= 24) skeletal electrons the lowest energy structures have central MnC2B9 icosahedra. However, for the corresponding rhenadicarbaborane CpReC2B9H11 the lowest energy structures have central non-icosahedral ReC2B9 deltahedra with two degree 6 vertices, one of which is occupied by the rhenium atom. The low-energy structures for the metalladicarbaboranes studied in this work relate to the preferences of transition metal atoms for degree 6 vertices but those of boron and carbon for degree 5 and 4 vertices, respectively.

 Please wait while we load your content...
Please wait while we load your content...