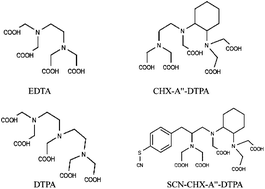
The in vivo212Pb/212Bi generator is promising for application in targeted alpha therapy (TAT) of cancer. One main limitation of its therapeutic application is due to potential release of 212Bi from the radioconjugate upon radioactive decay of the mother nuclide 212Pb, potentially leading to irradiation of healthy tissue. The objective of the present work is to assess whether the chelate CHX-A′′-DTPA (N-(2-aminoethyl)-trans-1,2-diaminocyclohexane-N,N′,N′′-pentaacetic acid) bound to a biological carrier molecule may be able to re-complex released 212Bi under in vivo conditions to limit its translocation from the target site. CHX-A′′-DTPA was bound to bovine gamma globulin (BGG) to mimic a model conjugate and the stability of the Bi-CHX-A′′-DTPA-BGG conjugate was studied in blood serum by ultrafiltration. TRLFS experiments using Cm(III) as a fluorescent probe demonstrated that linking CHX-A′′-DTPA to BGG does not affect the coordination properties of the ligand. Furthermore, comparable stability constants were observed between Bi(III) and free CHX-A′′-DTPA, BGG-bound CHX-A′′-DTPA and DTPA. The complexation constants determined between Bi(III) and the chelate molecules are sufficiently high to allow ultra trace amounts of the ligand to efficiently compete with serum transferrin controlling Bi(III) speciation in blood plasma conditions. Nevertheless, CHX-A′′-DTPA is not able to complex Bi(III) generated in blood serum because of the strong competition between Bi(III) and Fe(II) for the ligand. In other words, CHX-A′′-DTPA is not “selective” enough to limit Bi(III) release in the body when applying the 212Pb/212Bi in vivo generator.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...