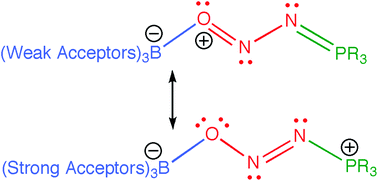
Computational studies of complexes Ar3B–ONN–PR3 derived from reactions between borane–phosphine frustrated Lewis pairs and N2O reveal several interesting facets. Natural resonance theory calculations support a change in the preferred resonance structure as the Lewis acidity of the borane increases. Potential constitutional isomers where phosphorus binds to oxygen and boron to nitrogen are predicted to be unstable with respect to loss of phosphine oxide and free N2. Other constitutional isomers represent stationary points on the potential energy surface; most are considerably less stable than the observed complexes, but one is predicted to be as stable. This arises because the dominant resonance form combines alternating charge with the presence of a stabilizing NO double bond. The relationship between Lewis acidity and complex formation for a variety of boranes was explored; the results are consistent with the idea that greater Lewis acidity stabilizes both classical and frustrated Lewis acid–base pairs, but to differing degrees such that both types can entrap N2O. Calculations addressing the mechanism of complex formation suggest that N2O binds first through the nitrogen to the phosphine phosphorus of the FLP, whereupon boron coordinates the oxygen atom. Studies of the mechanism of the degenerate exchange reaction between (4-F–H4C6)3B–ONN–P(t-Bu)3 and B(C6H4–4-F)3, involves a “transition state”, with relatively short B–O distances, and so resembles a classical Ia process. The process involves two barriers, one associated with bringing the incoming borane into proximity with the oxygen, and the other associated with isomerising from a ladle-shaped cis–trans ct conformer to the observed trans–trans tt-type structure. The overall barrier for degenerate exchange was predicted to be between 65 and 110 kJ mol−1, in fair agreement with experiment. Similar studies of the reaction between (4-F–H4C6)3B–ONN–P(t-Bu)3 and B(C6F5)3 indicate that this process more closely resembles a classical Id process, in that the “transition state” involves long B–O distances. Derivatization of the complexed NNO fragment appears possible; interaction between (F5C6)3B–ONN–P(t-Bu)3 and MeLi suggests stability for the ion pairs (F5C6)3B–ON(Me)N–P(t-Bu)3−/Li+ and (F5C6)3B–ONN(Me)–P(t-Bu)3−/Li+.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...