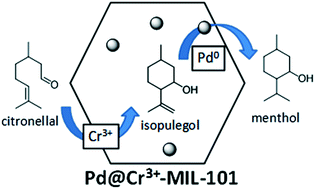
MOFs as multifunctional catalysts: One-pot synthesis of menthol from citronellal over a bifunctional MIL-101 catalyst†
Abstract
A bifunctional MOF
- This article is part of the themed collection: Coordination chemistry in the solid state

 Please wait while we load your content...
Please wait while we load your content...