Mechanochemical and solution synthesis, and crystal structures and IR and solid-state (CPMAS) NMR spectroscopy of some bis(triphenylphosphine)silver(i) mono- and di-hydrogencitrate systems†
Abstract
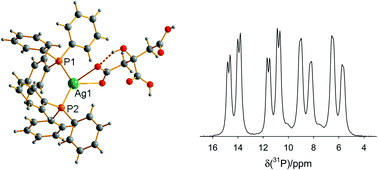
The complex [(Ph3P)2Ag(H2cit)]·EtOH (1; H2cit− = dihydrogencitrate = C6H7O7−) contains [(Ph3P)2Ag(H2cit)] molecules in which the silver atom is coordinated to two PPh3 molecules and the two oxygen atoms of one of the ‘terminal’/1-carboxylate groups of the dihydrogencitrate group. The molecules form centrosymmetric hydrogen-bonded dimers in the solid. In [{(Ph3P)2Ag}2(Hcit)], (2), unsymmetrical deprotonation of the citrate grouping is found, from the 1- and 3- (i.e. terminal and central) carboxylates: [(Ph3P)2Ag(O2CCH2C(OH) (CH2COOH)CO2)Ag(PPh3)2]. The above complexes, as well as [(Ph3P)3Ag(H2cit)] (3) were prepared via conventional solution methods, involving the reaction of

 Please wait while we load your content...
Please wait while we load your content...