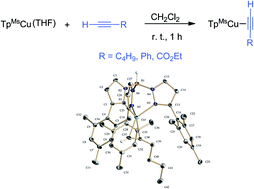
The use of the bulky hydrotris(3-mesitylpyrazolyl)borate anionic ligand has allowed the synthesis of stable TpMsCu(alkyne) complexes (alkyne = 1-hexyne, 1, phenylacetylene, 2, and ethyl propiolate, 3). The spectroscopic and structural features of these compounds and their relative reactivity have been examined, indicating the existence of a low π back-bonding from the copper(I) centre to the alkyne. Ligand exchange experiments have shown that terminal alkyne adducts are more stable than internal alkyne analogues. In good accordance with this, the previously reported alkyne cyclopropenation reaction catalysed by the TpxCu complexes can be rationalized and correlated with their relative stability.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...