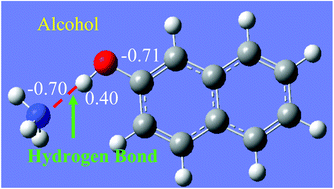
The adsorption of ammonia in four metal–organic frameworks modified with different functional groups (–OH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –Cl, –COOH) was investigated using a hierarchical molecular modeling approach. To describe the hydrogen bonding and other strong interactions between NH3 and the surface functional groups, a set of Morse potential parameters were obtained by fitting to energies from quantum chemical calculations at the MP2 level of theory. We describe a systematic force field parameterization process, in which the Morse parameters were fitted using simulated annealing to match a large number of single-point MP2 energies at various distances and angles. The fitted potentials were then used in grand canonical Monte Carlo simulations to predict ammonia adsorption isotherms and heats of adsorption in functionalized MIL-47, IRMOF-1, IRMOF-10, and IRMOF-16. The results show that ammonia adsorption can be significantly enhanced by using materials with appropriate pore size, strongly interacting functional groups, and high density of functional groups.
O, –Cl, –COOH) was investigated using a hierarchical molecular modeling approach. To describe the hydrogen bonding and other strong interactions between NH3 and the surface functional groups, a set of Morse potential parameters were obtained by fitting to energies from quantum chemical calculations at the MP2 level of theory. We describe a systematic force field parameterization process, in which the Morse parameters were fitted using simulated annealing to match a large number of single-point MP2 energies at various distances and angles. The fitted potentials were then used in grand canonical Monte Carlo simulations to predict ammonia adsorption isotherms and heats of adsorption in functionalized MIL-47, IRMOF-1, IRMOF-10, and IRMOF-16. The results show that ammonia adsorption can be significantly enhanced by using materials with appropriate pore size, strongly interacting functional groups, and high density of functional groups.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –Cl, –COOH) was investigated using a hierarchical molecular modeling approach. To describe the hydrogen bonding and other strong interactions between
O, –Cl, –COOH) was investigated using a hierarchical molecular modeling approach. To describe the hydrogen bonding and other strong interactions between 

 Please wait while we load your content...
Please wait while we load your content...