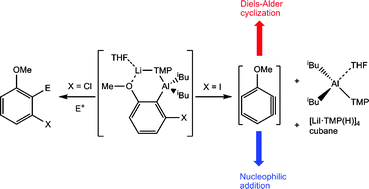
Gaining a deeper understanding of the modus operandi of heterometallic lithium aluminate bases towards deprotonative metallation of substituted aromatic substrates, we have studied the reactions and their aftermath between our recently developed bis-amido base ‘iBu2Al(μ-TMP)2Li’ 3 and 3-halogenated anisoles. Ortho-metallation of 3-iodoanisole with 3 results in a delicately poised heterometallic intermediate whose breakdown into homometallic species and benzyne cannot be suppressed, even at low temperature or in a non-polar solvent (hexane). Homometallic components [LiI·TMP(H)]4 (5) and iBu2Al(TMP)·THF (6) have been isolated while the reactive benzyne intermediate has been trapped via Diels–Alder cyclization with 1,3-diphenylisobenzofuran yielding 1-methoxy-9-10-diphenyl-9-10-epoxyanthracene (7). In polar THF solution, nucleophilic addition of LiTMP across the benzyne functionality followed by electrophilic quenching with iodine yields the trisubstituted aromatic species 1-(2-iodo-3-methoxyphenyl)-2,2,6,6-tetramethylpiperidide (8). Compounds 5–8 have been characterized by single-crystal X-ray diffraction in the solid state and multinuclear NMR spectroscopy in solution. By considering these collated results, a plausible reaction mechanism has been proposed for the breakdown of the aforementioned intermediate bimetallic framework. Interestingly, the metallation reaction can be controlled by changing to 3-chloroanisole with an excess of base 3, as evidenced by electrophilically trapping the deprotonated aromatic with iodine to give 2-iodo-3-chloroanisole (9).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...