Co-ordination behaviour of a novel bisthiourea tripodal ligand: structural, spectroscopic and electrochemical properties of a series of transition metal complexes†
Abstract
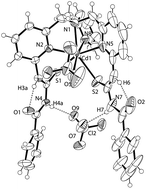
The synthesis of a thiourea substituted derivative of tris(pyridyl-2-methyl)amine (TPA) is reported. Two of the three pyridine rings are substituted in the 6-position with benzoylthiourea groups. These thiourea groups undergo intramolecular hydrogen bonding to form six-membered rings which leaves one N–H group available to form hydrogen bonds with other molecules. This reports details how the complexation of this new

 Please wait while we load your content...
Please wait while we load your content...