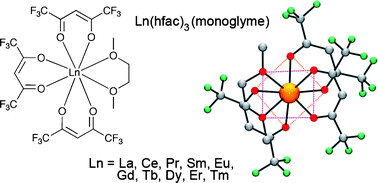
Syntheses and crystal structures of anhydrous Ln(hfac)3(monoglyme). Ln = La, Ce, Pr, Sm, Eu, Gd, Tb, Dy, Er, Tm†
Abstract
The crystal structures of a broad series of anhydrous Ln(hfac)3(monoglyme) complexes, prepared in moderate to high yield, are presented: hfac = 1,1,1,5,5,5-hexafluoroacetylacetonato-; Ln = La, Ce, Pr, Sm, Eu, Gd, Tb, Dy, Er, Tm. This study contradicts the general assumption that

 Please wait while we load your content...
Please wait while we load your content...