Non-enzymatic dynamic kinetic resolution of racemic α-arylalkanoic acids: an advanced asymmetric synthesis of chiral nonsteroidal anti-inflammatory drugs (NSAIDs)†
Abstract
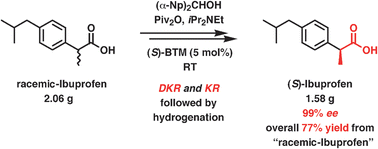
An efficient protocol was developed to produce chiral 2-arylalkanoic esters in high yields (up to 99%) from racemic

 Please wait while we load your content...
Please wait while we load your content...