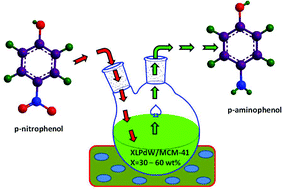
A green and effective method is reported for the hydrogenation of p-nitrophenol to p-aminophenol using a Cs salt of Pd substituted keggin-type monolacunary phosphotungstate supported mesoporous silica (LPdW/MCM-41). A sample containing 50 wt% loading of the Pd substituted lacunary salt gave the best result towards the reaction, i.e. 99% conversion and 100% p-aminophenol selectivity. This economical and environmentally friendly method carried out at room temperature provides a potentially new approach for the synthesis of p-aminophenol from p-nitrophenol. In addition, the possibility to reuse the catalyst many times with great efficiency is another advantage. The catalyst was characterized by different techniques like X-ray diffraction, N2 adsorption–desorption, scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), phosphorous-31 NMR spectroscopy (31P NMR), Raman spectra and Fourier-Transform infrared spectroscopy (FT-IR). FT-IR studies confirmed the undegraded lacunary keggin structure of the salt supported on MCM-41.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...