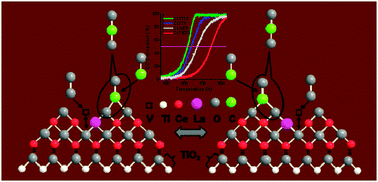
Titania supported ceria–lanthana solid solutions (CexLa1−xO2−δ/TiO2; CLT) have been synthesized by a facile and economical route. Existence of synergism between ceria–lanthana (CL) solid solutions and titania-anatase phase, which leads to decrease in the crystallite size, retarded titania phase transformation, and improved redox properties, has been thoroughly investigated by various techniques, namely, X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM), UV-visible diffuse reflectance spectroscopy (UV–vis DRS), Raman spectroscopy (UV–RS and Vis–RS), BET surface area analysis, and temperature programmed reduction (TPR). Two key observations made from the whole exercise were (i) mutual interaction of Ce and Ti ions could impose typical Ce–O–Ti modes at the interfacial region and (ii) the La3+ ion as a dopant provokes a large number of oxygen vacancies via a charge compensation mechanism. The promising role of these factors in the CO oxidation (one of the most formidable challenges) has been comprehensively described. The observed enhanced activity for the CLT sample is primarily attributed to an apparent specific orientation of the active component over the support, which is endorsed by the interfacial interaction. This specific mode could facilitate the CO adsorption with simultaneous bulk oxygen diffusion for more consumption and in turn better activity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...