Theoretical investigation on copper hydrides catalyzed hydrosilylation reaction of 3-methylcyclohex-2-enone: mechanism and ligands' effect†
Abstract
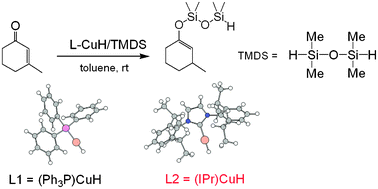
The mechanism of the hydrosilylation reactions of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in the substrate, and the regeneration of the copper hydrides assisted by TMDS. The calculations indicate that the
C bond in the substrate, and the regeneration of the copper hydrides assisted by TMDS. The calculations indicate that the

 Please wait while we load your content...
Please wait while we load your content...