Enantioselective, transition metal catalyzed cycloisomerizations
Abstract
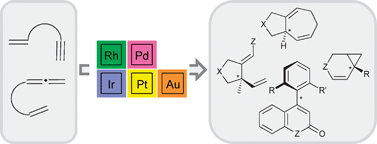
This review illustrates enantioselective transition-metal promoted skeletal rearrangements of polyunsaturated substrates possessing olefin, alkyne or allene functions. These processes are classified according to the number of carbon atoms involved in the cyclization, from (1C+1C) to (2C+2C+2C) or (2C+5C) cyclizations. Thus, for instance, (1C+1C) processes are typified notably by Alder-ene type reactions taking place mainly under palladium and rhodium catalysis, in the presence of chiral phosphorus ligands. Also, rhodium, platinum, and gold promoted insertions of unsaturated carbon–carbon bonds into C–H bonds belong to this class. For each class of reactions or substrate type the best ligand–metal pairs are highlighted. Unfortunately, unlike other transition metal promoted reactions, the mechanisms of chiral induction and stereochemical pathways have not been established so far in any of these reactions. In only a few instances, qualitative heuristic models have been tentatively proposed. Although the available stereochemical information is systematically given here, the paper focuses mainly on synthetic aspects of enantioselective cycloisomerizations.

 Please wait while we load your content...
Please wait while we load your content...