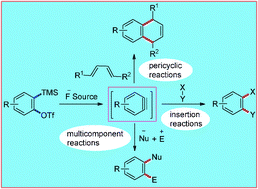
This tutorial review is aimed at highlighting recent developments in transition-metal-free carbon–carbon and carbon–heteroatom bond-forming reactions utilizing a versatile class of reactive intermediates, viz., arynes, which hold the potential for numerous applications in organic synthesis. Key to the success of the resurgence of interest in the rich chemistry of arynes is primarily the mild condition for their generation by the fluoride-induced 1,2-elimination of 2-(trimethylsilyl)aryl triflates. Consequently, arynes have been employed for the construction of multisubstituted arenes with structural diversity and complexity. The versatile transition-metal-free applications of arynes include cycloaddition reactions, insertion reactions and multicomponent reactions. In addition, arynes have found applications in natural product synthesis. Herein, we present a concise account of the major developments that occurred in this field during the past eight years.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...