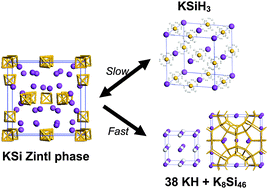
The recently reported KSi–KSiH3 system can store 4.3 wt% of hydrogen reversibly with slow kinetics of several hours for complete absorption at 373 K and complete desorption at 473 K. From the kinetics measured at different temperatures, the Arrhenius plots give activation energies (Ea) of 56.0 ± 5.7 kJ mol−1 and 121 ± 17 kJ mol−1 for the absorption and desorption processes, respectively. Ball-milling with 10 wt% of carbon strongly improves the kinetics of the system, i.e. specifically the initial rate of absorption becomes about one order of magnitude faster than that of pristine KSi. However, this fast absorption causes a disproportionation into KH and K8Si46, instead of forming the KSiH3 hydride from a slow absorption. This disproportionation, due to the formation of stable KH, leads to a total loss of reversibility. In a similar situation, when the pristine Zintl NaSi phase absorbs hydrogen, it likewise disproportionates into NaH and Na8Si46, indicating a very poorly reversible reaction.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...