New results for the formation of a muoniated radical in the Mu + Br2 system: a van der Waals complex or evidence for vibrational bonding in Br–Mu–Br?
Abstract
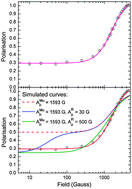
New evidence is presented for the observation of a muoniated radical in the Mu + Br2 system, from μSR longitudinal field (LF) repolarisation studies in the gas phase, at Br2 concentrations of 0.1 bar in a Br2/N2 mixture at 300 K and at 10 bar total pressure. The LF repolarisation curve, up to a field of 4.5 kG, reveals two paramagnetic components, one for the Mu atom, formed promptly during the slowing-down process of the ![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) –
–![[L with combining low line]](https://www.rsc.org/images/entities/i_char_004c_0332.gif) –
–![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) system, or of a Mu⋯Br2 van der Waals complex formed in the entrance channel. Preliminary ab initio electronic structure calculations suggest the latter is more likely but fully rigorous calculations of the effect of dynamics on the hfcc for either system have yet to be carried out.
system, or of a Mu⋯Br2 van der Waals complex formed in the entrance channel. Preliminary ab initio electronic structure calculations suggest the latter is more likely but fully rigorous calculations of the effect of dynamics on the hfcc for either system have yet to be carried out.

 Please wait while we load your content...
Please wait while we load your content...