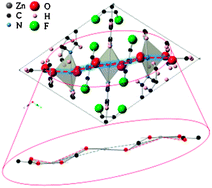
Van der Waals forces in the perfluorinated metal–organic framework zinc 1,2-bis(4-pyridyl)ethane tetrafluoroterephthalate
Abstract
Traditional density functional theory (DFT) and dispersion-corrected DFT calculations are performed to investigate the metal–organic framework

 Please wait while we load your content...
Please wait while we load your content...