CO2 capture in aqueous ammonia solutions: a computational chemistry perspective†
Abstract
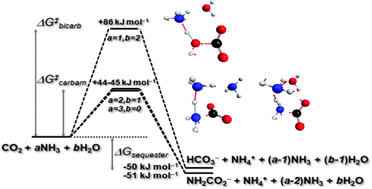
Twenty-five transition structures (TS's) for CO2 fixation by up to four base molecules (ammonia or ammonia + water) were located using M06-2X/6-311++G(d,p). All lead to either carbamate (NH2CO2−) or bicarbonate (HCO3−) products. Single-point energies at CCSD(T)/maug-cc-pVTZ//M06-2X/6-311++G(d,p) were added to SM8/M06-2X/6-311++G(d,p) energies to obtain best-estimate aqueous activation energies. All theories agree that: (i) NH2CO2− formation has a lower free energy of activation (best est. 44–45 kJ mol−1) than HCO3− formation (best est. 86 kJ mol−1), and (ii) free energies of activation for CO2 fixation are lowered when an ammonia molecule accepts the proton from the nucleophilic base. The theory also supports a key role for ammonium ions in the observed decomposition of NH2CO2− near pH 9.

 Please wait while we load your content...
Please wait while we load your content...