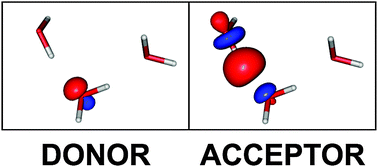
Using the ωB97X-D and B3LYP density functionals, the absolutely localized molecular orbital energy decomposition method (ALMO-EDA) is applied to the water dimer through pentamer, 13-mer and 17-mer clusters. Two-body, three-body, and total interaction energies are decomposed into their component energy terms: frozen density interaction energy, polarization energy, and charge transfer energy. Charge transfer, polarization, and frozen orbital interaction energies are all found to be significant contributors to the two-body and total interaction energies; the three-body interaction energies are dominated by polarization. Each component energy term for the two-body interactions is highly dependent on the associated hydrogen bond distance. The favorability of the three-body terms associated with the 13- and 17-mer structures depends on the hydrogen-donor or hydrogen-acceptor roles played by each of the three component waters. Only small errors arise from neglect of three-body interactions without two adjacent water molecules, or beyond three-body interactions. Interesting linear correlations are identified between the contributions of charge-transfer and polarization terms to the two and three-body interactions, which permits elimination of explicit calculation of charge transfer to a good approximation.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...