Novel group 13 Lewis superacids and 13–15 donor–acceptor cryptands for hydrogen activation: a theoretical study†
Abstract
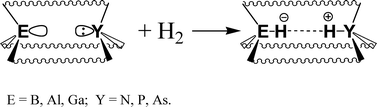
Structures of group 13 Lewis acids ER3 and group 15 Lewis bases YR′3 as well as respective hydrogen splitting products [HER3]− and [HYR′3]+ (E = B, Al, Ga; Y = N, P, As; R3 = (C6F5)3, (C6H2F2)3CF; R′ = H, C6H5, 2,6-Me2C6H3) have been fully optimized at B3LYP/pVDZ and B3LYP/def2-TZVPP levels of theory. Energetic characteristics of the hydrogen-splitting process ER3 + YR′3 + H2 = [HYR′3]+ + [HER3]− have been obtained. Energetics for the ion formation does not depend on the nature of group 13 element E. However, the Lewis acid environment (pyramidal vs. planar) and nature of Lewis base play a significant role in the energetics of heterocyclic hydrogen splitting. The endothermicity of the heterolytic hydrogen splitting process is reduced by about 80 kJ mol−1 by using pyramidal Lewis acids E(C6H2F2)3CF, and by 40 kJ mol−1 by using Lewis bases with 2,6-Me2C6H3 substituents. Thus, the overall process of the heterolytic hydrogen splitting is predicted to be endothermic by only 130 kJ mol−1 for the E(C6H2F2)3CF–P(2,6-Me2C6H3)3 combinations (compared to 450 kJ mol−1 for E(C6F5)3–PH3). It is shown that H2 splitting in the gas phase by nitrogen-containing DA cryptands, featuring group 13 element acceptor centers with a pyramidalized environment, is highly exothermic.
- This article is part of the themed collection: Predicting new molecules by quantum chemical methods

 Please wait while we load your content...
Please wait while we load your content...