Strong interactions between copper halides and unsaturated systems: new metallocycles? Or the importance of deformation†
Abstract
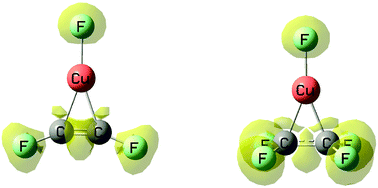
The complexes formed by CuF with CC double and triple bonds have been studied at the MP2 and CCSD(T) computational levels. The interaction of CuF with acetylene, ethylene and their fluoro derivatives is very strong, with interaction energies close to those of conventional covalent bonds. Hence, these complexes could be actually viewed as a new kind of metallocycles, with significantly strong Cu–C linkages. All electronic indexes analyzed by means of the AIM, ELF and NBO formalisms, indicate that the strength of the interaction should increase with the number of fluorine substituents in both series of compounds. Surprisingly, however, although both series of compounds exhibit the same bonding arrangements, they follow opposite stability trends and the expected increase of the interaction energies with the number of fluorine substituents is only observed in the acetylene series. The reason for this unexpected behavior is once more associated with the effects triggered by the distortion of the interacting subunits. Deformation not only has a direct energetic cost but dramatically affects the intrinsic properties of the interacting systems.

 Please wait while we load your content...
Please wait while we load your content...