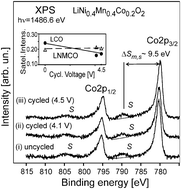
The stability of the valence state of the 3d transition metal ions and the stoichiometry of LiMO2 (M = Co, Ni, Mn) layered oxides at the surface–electrolyte interface plays a crucial role in energy storage applications. The surface oxidation/reduction of the cations caused by the contact of the solids to air or to the electrolyte results in the blocking of the Li-transport through the interface that leads to the fast batteries deterioration. The influence of the end-of-charge voltage on the chemical composition and the oxidation state of 3d transition metal ions, as well as the stability of the solid–electrolyte interface formed during the electrochemical Li-deintercalation/intercalation of the LiCoO2 and Li(Ni,Mn,Co)O2, have been investigated by X-ray photoelectron spectroscopy. While the chemical composition of the solid–electrolyte interface is similar for both layered oxide surfaces, the electrochemical cycling to some critical voltage values leads to the disappearance of the interface. By the analysis of the shape of the 2p and 3s photoelectron emissions we show that the formation of the solid–electrolyte interface layer correlates with the partial reduction of the trivalent Co ions at the electrolyte–LiCoO2 interface and the amount of the Co2+ ions is increased as the solid–electrolyte interface vanishes. In contrast, the Mn4+, Co3+ and Ni2+ ions of the Li(Ni,Mn,Co)O2 are stable at the interface under the electrochemical cycling to higher end-of-charge voltage. A correlation between deterioration of the LiCoO2 and Li(Ni,Mn,Co)O2 batteries and the change of electronic structure at the surface/interface after the electrochemical cycling has been found. The dissolution of the solid–electrolyte interface layer might be the reason for the fast deterioration of the Li-ion batteries.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...