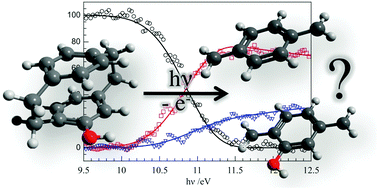
The vacuum ultraviolet (VUV) photoionization and dissociative photoionization of three hydroxy-substituted [2.2]paracyclophane derivatives were studied yielding adiabatic ionization energies and dissociative photoionization energies (appearance energies). The simplest dissociation pathway is the breaking of both CH2–CH2 bridge units and fragmenting the molecular ion in half to yield xylylene neutral and cationic fragments. The experimental data show that this process is outcompeted by a faster, higher energy channel, possibly yielding cyclooctatetraene derivatives. The role of the reaction coordinate, the effect of large amplitude motions on the density of states function at low and high energies and the temperature dependent ‘population gap’ in the internal energy distribution in large molecules are discussed in the context of applying statistical models to the dissociation. Computational approaches to the binding energy of paracyclophanes are marred with pitfalls. Noncovalent interactions play a major role in keeping paracyclophanes bound by some 200 kJ mol−1 with respect to the two xylylene motifs, and the covalent CH2–CH2 bonds are mostly counteracted by the geometric strain. The stabilizing effects are twofold: first, paracyclophanes are aromatic compounds, whereas xylylenes are not. Thus, the aromaticity of the molecule is induced by dimerization. Second, dispersive π–π interactions also stabilize the molecule. We evaluated 23 different computational chemistry approaches, and found that very few of the favorably scaling ones give an adequate description of this system. Among the DFT functionals tested, only PBE-D3 and perhaps M06-2X yielded consistently accurate results, comparable with MP3 and CCSD, or the G4 and CBS-QB3 composite methods. MP2 results in general suffer from significant overbonding.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...