Structure and stability of hexa-aqua V(iii) cations in vanadium redox flow battery electrolytes
Abstract
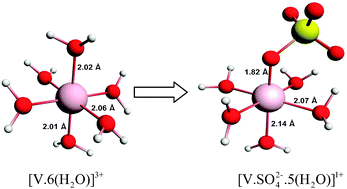
The vanadium(III) cation structure in mixed acid based electrolyte solution from vanadium redox flow batteries is studied by 17O and 35/37Cl nuclear magnetic resonance (NMR) spectroscopy, electronic spectroscopy and density functional theory (DFT) based computational modelling. Both computational and experimental results reveal that the V(III) species can complex with counter anions (sulfate/chlorine) depending on the composition of its solvation sphere. By analyzing the powder precipitate it was found that the formation of sulfate complexed V(III) species is the crucial process in the precipitation reaction. The precipitation occurs through nucleation of neutral species formed through deprotonation and ion-pair formation process. However, the powder precipitate shows a multiphase nature which warrants multiple reaction pathways for precipitation reaction.

 Please wait while we load your content...
Please wait while we load your content...