Microchemical inhomogeneity to characterize atomic configurations in the heating and quenching of a CuHf2 alloy
Abstract
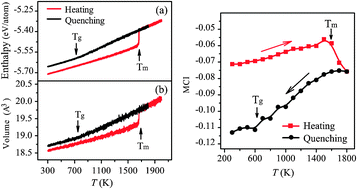
Based on the constructed Cu–Hf interatomic potential, Monte Carlo simulations were conducted to reveal the atomic configurations in heating and quenching of a CuHf2 alloy through scrutinizing the evolution of microchemical inhomogeneity. Simulations show that the CuHf2 crystalline structure becomes more homogeneous during heating but an obvious drop in microchemical inhomogeneity appears when reaching its melting point. During the quenching process, the CuHf2 melt becomes increasingly inhomogeneous and shows a change in the slope in the microchemical inhomogeneity around glass transition temperature. Simulation results were evidenced by the atomic packing analysis through the Voronoi tessellation method. The implications of our study suggest that the glass transition could be visualized as a process involving increase of microchemical inhomogeneity from the liquid to glassy state.

 Please wait while we load your content...
Please wait while we load your content...