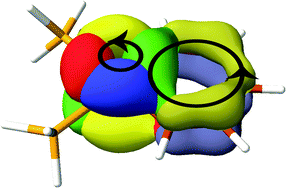
Ring currents calculated in the ipsocentric CTOCD-DZ formalism are presented for four representative metallabenzenes, compounds in which a benzene CH group is formally replaced by a transition metal atom with ligands. Aromaticity is probed using ring currents computed using non-relativistic and relativistic orbitals (derived with relativistic effective core potentials or ZORA). Maps computed at different levels of relativistic theory turn out to be similar, showing that orbital nodal character is the main determinant of ring current. Diatropic/paratropic global ring currents in these compounds, and also circulations localised on the metal centre, are interpreted in terms of contributions of localised π-type orbitals and metal d-orbitals, respectively. All four considered metallabenzenes should be regarded as 6π electron species, despite the fact that three support diatropic (‘aromatic’) ring currents and one a paratropic (‘anti-aromatic’) current. The current-density maps determine the correct way to count electrons in these species: differential occupation of d-orbitals of formal π-symmetry contributes to circulation on the metal centre, but not around the benzenoid ring. The overall trend from strongly diatropic to weakly paratropic ring currents along the series 1 to 4 is explained by the increasing strength of interaction between formally non-bonding orbitals on the metal centre and C5H5 moiety, which together make up the six-membered ring.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...