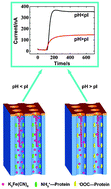
A nanochannel array based nanodevice can mimic the biological environments and thus unveil the natural properties, conformation and recognition information of biomolecules such as proteins and DNA in confined spaces. Here we report that porous anodic alumina (PAA) of a highly parallel nanochannel array covalently modified with proteins significantly modulates the transport of a negatively charged probe of ferricyanide due to the electrostatic interactions between the probes and modified nanochannel inner surface. Results show that such electrostatic interaction exists in a wide range of ionic strength from 1 mM to 100 mM in 20 nm nanochannels modified with proteins (hemoglobin, bovine serum albumin, and goat anti-rabbit IgG secondary antibody). In addition, the maximal steady-state flux of the charged probe through the modified nanochannel array is directly related to the ionic strength which determines the electric double layer thickness and solution pH which modulates the nanochannel surface charge. Thus, the modulated mass transport of the probe by solution pH can be used to study the charge properties of the immobilized proteins in nanochannel confined conditions, leading us to obtain the isoelectric point (pI) of the proteins confined in nanochannels. The determined pI values of two known proteins of hemoglobin and bovine serum albumin are close to the ones of the same proteins covalently modified on a 3-mercaptopropionic acid self-assembled monolayer/gold electrode. In addition, the pI of an unknown protein of goat anti-rabbit IgG secondary antibody confined in nanochannels was determined to be 6.3. Finally, the confinement effect of nanochannels on the charge properties of immobilized proteins has been discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...