Does cation dehydration drive the binding of metal ions to polyelectrolytes in water? What we can learn from the behaviour of aluminium(iii) and chromium(iii)†
Abstract
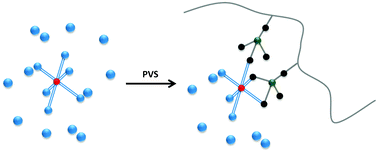
Much stronger binding is seen in aqueous solutions between the anionic polyelectrolyte potassium poly(vinyl sulfate) and the substitution labile aluminium(III) than with the kinetically inert chromium(III). This strongly supports the idea that entropy driven

 Please wait while we load your content...
Please wait while we load your content...