Experimental observation of preferential solvation on a radical ion pair using MARY spectroscopy
Abstract
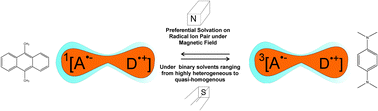
The effect of preferential solvation on the exciplex luminescence detected magnetic field effect has been studied using magnetic-field-effect-on-reaction-yield (MARY)

 Please wait while we load your content...
Please wait while we load your content...