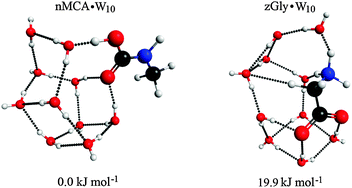
We have theoretically investigated how the low-energy conformers of the neutral and the zwitterionic forms of glycine as well as methylcarbamic acid are stabilized by the presence water. The MP2/6-311++G(d,p) method was utilized to conduct calculations on glycine and methylcarbamic acid in both isolated clusters and in clusters embedded in the conductor-like polarizable continuum model (C-PCM), where the clusters explicitly contain between one and ten water molecules. The neutral forms of glycine and methylcarbamic acid were found to have similar hydration energies, whereas the neutral methylcarbamic acid was determined to be approximately 32 kJ mol−1 more stable than the neutral glycine in the isolated clusters and 30 kJ mol−1 more stable in the C-PCM embedded clusters. Both the number and strength of the hydrogen bonding interactions between water and the zwitterions drive the stability. This lowers the relative energy of the glycine zwitterion from 50 kJ mol−1 above neutral glycine, when there are two water molecules in the clusters to 11 kJ mol−1 below for the clusters containing ten water molecules. For the methylcarbamic acid clusters with two water molecules, the zwitterion is 51 kJ mol−1 higher in energy than the neutral form, but it remains 13 kJ mol−1 above the neutral methylcarbamic acid in the clusters containing ten water molecules. When the bulk water environment is simulated by the C-PCM calculations, we find both the methylcarbamic acid and glycine zwitterionic forms have similar energies at 20 kJ mol−1 above the neutral methylcarbamic acid energy and 10 kJ mol−1 lower than the neutral glycine energy. Although neither methylcarbamic acid nor glycine have been detected in the interstellar medium yet, our findings indicate that methylcarbamic acid is the more stable product from methylamine and carbon dioxide reactions in a water ice. This suggests that methylcarbamic acid likely plays a role in the intermediate steps if glycine is formed in the interstellar medium.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...