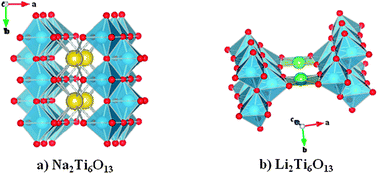
A detailed structural and electrochemical study of the ion exchanged Li2Ti6O13 titanate as a new anode for Li-ion batteries is presented. Subtle structural differences between the parent Na2Ti6O13, where Na is in an eightfold coordinated site, and the Li-derivative, where Li is fourfold coordinated, determine important differences in the electrochemical behaviour. While the Li insertion in Na2Ti6O13 proceeds reversibly the reaction of lithium with Li2Ti6O13 is accompanied by an irreversible phase transformation after the first discharge. Interestingly, this new phase undergoes reversible Li insertion reaction developing a capacity of 170 mAh g−1 at an average voltage of 1.7 V vs. Li+/Li. Compared with other titanates this result is promising to develop a new anode material for lithium ion rechargeable batteries. Neutron powder diffraction revealed that Na in Na2Ti6O13 and Li in Li2Ti6O13 obtained by Na/Li ion exchange at 325 °C occupy different tunnel sites within the basically same (Ti6O13)2− framework. On the other hand, electrochemical performance of Li2Ti6O13 itself and the phase released after the first full discharge is strongly affected by the synthesis temperature. For example, heating Li2Ti6O13 at 350 °C produces a drastic decrease of the reversible capacity of the phase obtained after full discharge, from 170 mAh g−1 to ca. 90 mAh g−1. This latter value has been reported for Li2Ti6O13 prepared by ion exchange at higher temperature.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...