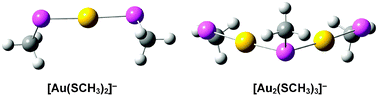
Thiolate-protected gold nanoparticles have been found recently to be coordinated by the so-called “staple” bonding motifs, consisting of quasi-linear [RS–Au–SR] and V-shaped [RS–Au–(SR)–Au–SR] units, which carry a negative charge formally. Using photoelectron spectroscopy (PES) in conjunction with ab initio calculations, we have investigated the electronic structure and chemical bonding of the simplest staples with R = CH3: Au(SCH3)2− and Au2(SCH3)3−, which were produced by electrospray ionization. PES data of the two Au–thiolate complexes are obtained both at room temperature (RT) and 20 K. The temperature-dependent study reveals significant spectral broadening at RT, in agreement with theoretical predictions of multiple conformations due to the different orientations of the –SCH3 groups. The Au–S bonds in Aun(SCH3)n+1− (n = 1, 2) are shown to be covalent via a variety of chemical bonding analyses. The strong Au–thiolate bonding and the stability of the Au–thiolate complexes are consistent with their ubiquity as staples for gold nanoparticles and on gold surfaces.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...