Theory of electron localization and its application to blue-shifting hydrogen bonds†
Abstract
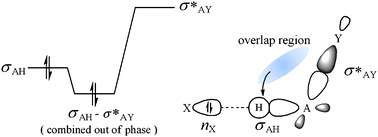
We propose a theory of electron localization or stabilization by electron localization through the interactions between occupied (i) and vacant (j*) orbitals under certain conditions, which have been believed so far to cause only electron delocalization. Electrons localize when the electrons redistributed by the interaction are more stable in the i-th occupied orbital than in the overlap region: hij* > sij*hii for sij* > 0. Electron delocalization occurs when hij* < sij*hii for sij* > 0. The hij* and sij*hii terms represent the energy of the electrons in the overlap region and the energy of the redistributed electrons in the occupied orbital, respectively. The theory of electron localization is substantiated by the correlation of the C–H bond lengths of fluorinated methanes H4–nCFn (n = 1, 2, 3) to the electron population of the σCH bonding orbital, and successfully applied to understanding blue-shifting hydrogen bonds in F3CH⋯X (X = CO, N2, OC, Ne, OC(CH3)2) and designing some

 Please wait while we load your content...
Please wait while we load your content...